
Counterfeit medicines and unauthorized product diversions remain one of the most dangerous threats to global public health.
Tracenac is a secure, AI-enabled serialization and product verification engine built to ensure full traceability, authentication, and regulatory compliance across your pharmaceutical supply chain.
Designed for manufacturers, CDMOs, exporters, and distributors, Tracenac delivers end-to-end serialization, real-time QR-based authentication, and geo-fenced anti-counterfeiting infrastructure—aligned with DSCSA, EU FMD, CDSCO, and WHO-GS1 compliance frameworks.
We offer a comprehensive suite of services to guide your brand through the complexities of the market.
> Generate tamper-proof, GS1-standard GTIN-embedded dynamic QR codes
> Include batch number, expiry, serial ID, manufacturing site, and API source
> QR codes are protected via blockchain hash signatures or device-bound fingerprinting
> Detect anomalies via scan frequency, geo-location, device ID, and route origin
> Trigger real-time alerts for potential diversion, tampering, or gray market infiltration
> Machine learning models evolve with new threats and counterfeit patterns
> Every product event (packaging, dispatch, scan, verification) is immutably logged
> Blockchain layer ensures compliance with 21 CFR Part 11, Annex 11, and DSCSA verification
> Tamper-evident trails support regulatory inspections, recalls, and export validations
> Immutable audit logs ensure tamper-proof traceability across GMP and CRO systems, compliant with 21 CFR Part 11, EU Annex 11, and GAMP® 5.
> AI proactively flags non-conformities against GxP, ICH E6(R2), and ISO 13485 to prevent Form 483 or MHRA inspection observations.
> Automated inspection readiness reports compile validated summaries like BMRs, CAPA logs, and VMPs, aligned with FDA, EMA, and CDSCO audit standards.
> Restrict scans to defined geographical zones, IP ranges, or authorized devices
> Identify unauthorized access attempts, clone scans, or excessive usage in blacklisted zones
> Ideal for regional SKU controls, international distribution audits, and post-market surveillance
> Public-facing portals (web/app) for patient, doctor, or distributor verification
> Enterprise-level dashboard to validate product lineage, logistics history, and distribution integrity
> Integrates with B2B customer portals, e-pharmacy APIs, or point-of-sale validation layers
Randomisation, packaging-level traceability, and export-ready QR validation
Verify authenticity across borders, prevent product swaps and refilling
Authenticate stock during inward receipt or before patient dispensation
Perform batch lineage tracebacks, recall verification, and enforcement support

> US FDA Drug Supply Chain Security Act (DSCSA)
> EU Falsified Medicines Directive (FMD)
> India CDSCO Track & Trace Mandate for Exports
> WHO GSDP Serialization & Labelling Guidelines
> GS1 GTIN, SGTIN, and EPCIS Event Architecture

> 100% randomized for regulated markets (US, EU, India, Africa, LATAM)
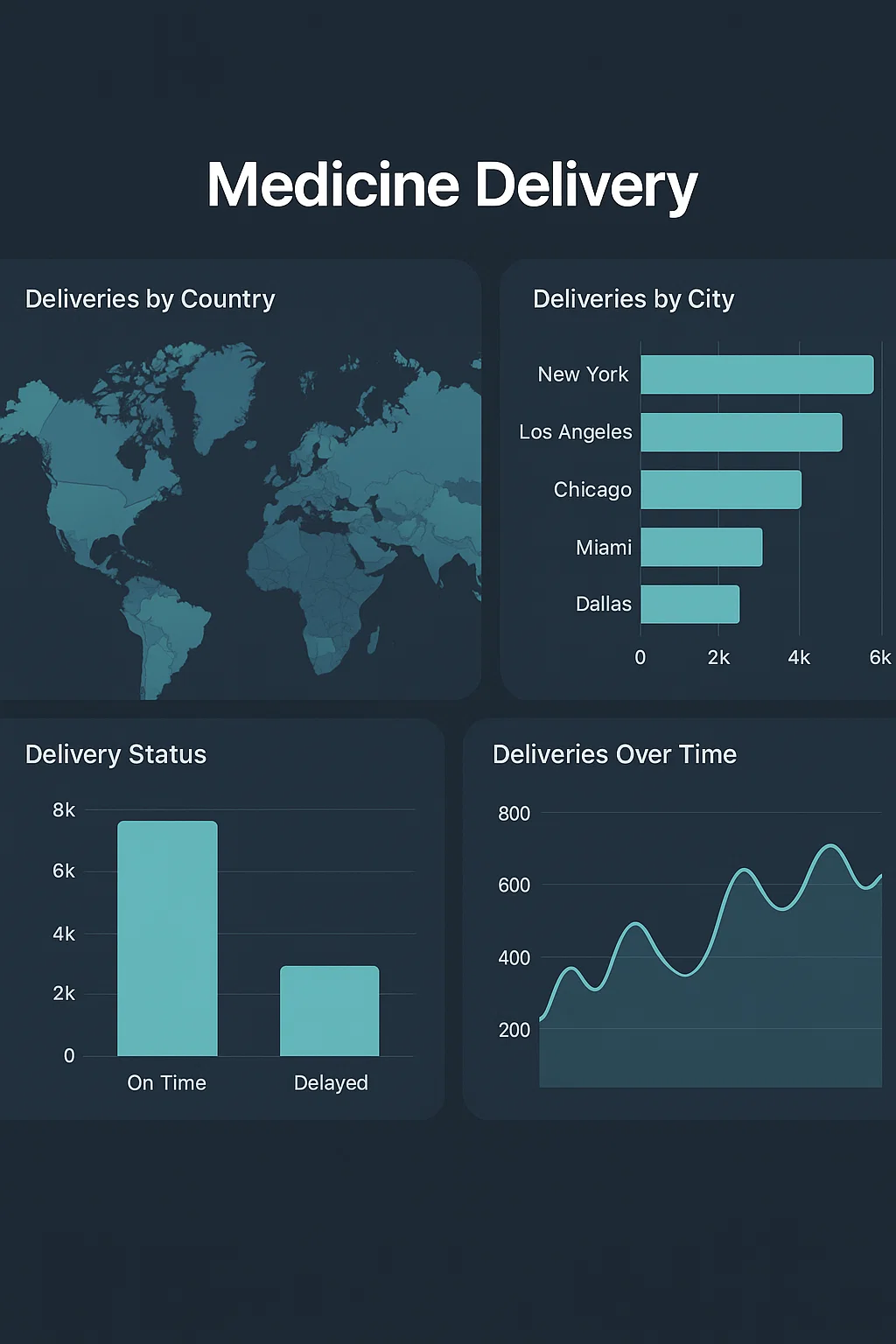
> 90–99% counterfeit incident reduction across pilot deployments
> Over 1 billion unique QR codes generated and verified
> Real-time insights into product movement, recall risk, and scan hotspots
What Makes Tracenac Unique?

